UK G4-1 for Solid Tumor Treatment
- Right to license and SRA to study 30-day survival in mice vs. competitor drugs
- Right to license lasts until 45 days after results reported to us under SRA (extendable)
- Computer-designed drug isolated from ~340,000 candidates
- Effective against solid tumors, unlike competitors

- Excellent metabolic stability profile relative to competitors
- Works in cancers already resistant to competitor drugs
- Patent subject to our Option expires 2035
- Synthesis of drug to use in 30-day study almost complete
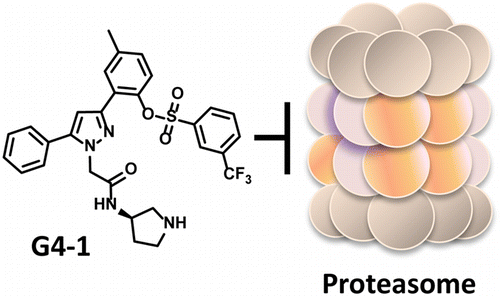
G4-1 - Drug designed to bind to proteosome
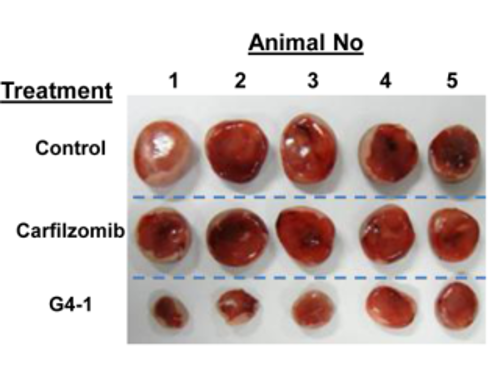
G4-1 inhibits tumor growth better than FDA-approved carfilzomib
Source: https://pubs.acs.org/doi/10.1021/jm501344na
Miller et al. (2015) J. Med. Chem 68:2036-41
Miller et al. (2015) J. Med. Chem 68:2036-41
Other Programs
Silo Pharma – PsylocybinTreatment in Cancer Patients
- Silo recently granted us a worldwide exclusive, sublicensable, royalty-bearing license to four patent applications relating to delivering psilocybin directly to neuroinflamedtissue
- Field of use includes “treatment of cancer and symptoms caused by cancer, including but not limited to pain, nausea, neuroinflammation, brain and neural dysfunction, depression, seizures, confusion, dizziness, numbness/tingling, dysfunction of the senses and all other symptoms that are caused by cancer of any type.”
- Currently seeking partners in academia to test in animal models.
Wake Forest’s KPC34 for AML and ALL
- Licensed to KPC34 for treatment of Acute Myeloid Leukemia (AML) and Acute Myeloid Leukemia (ALL)
- Small target patient populations: AML around 21K new/yr and ALL around 6K/yr
- Eligible for Orphan Drug Designation, providing 7 years market exclusivity
- KPC34 also based on gemcitabine, like UT’s DHA-dFdC
