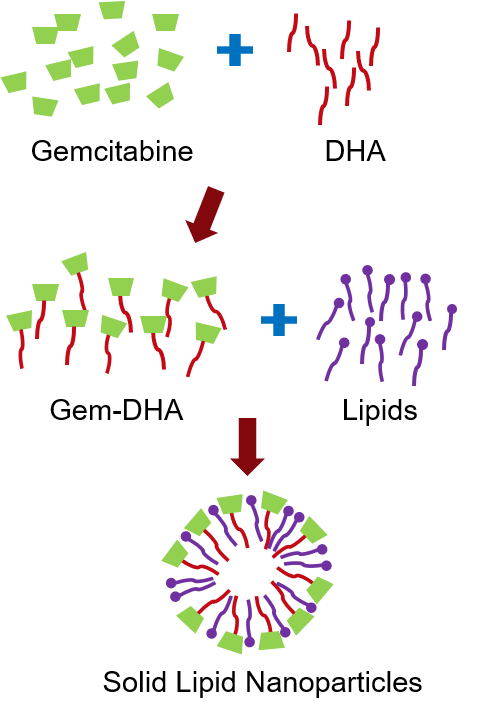
DHA-dFdC (Gem-DHA) for Pancreatic Cancer
Licensed to drug and a potentially next-generation oral delivery system
- Modified gemcitabine with robust data in mice
- Concentrates in pancreas
- Higher activity than gemcitabine
- Promising safety profile
- Greatly reduces drug resistance

- R&D Progressing
- Product produced at high yield
- Scale-up being validated
- Batch-to-batch testing underway
- Next steps
- Formulation development
- GMP manufacturing protocol
- Pre-IND animal studies
Ongoing Work:
- Collaborating with ParimerScientific in South Carolina to manufacture drug to FDA quality
- Site certified for ISO 9001 quality control
- Manufacture of DHA-dFdCas taught in literature successfully replicated
- Product produced at high yield
- Scale up manufacturing procedure currently being validated
- Manufacture of small batch for trial testing currently underway
- Dose formulation development to begin thereafter; both oral and IV formulations pursued in parallel
- Limited animal testing to begin in the latter stages of formulation development
- Will hire on contract research organization (CRO) for animal testing soon
