Dual Radiotherapy for Prostate Cancer
-
Ownership interest in Convergent Therapeutics, Inc., licensed by Weill Cornell Medicine
- Form 8-K filed 2/3/2021 at https://ir.aikidopharma.com/sec-filings/
- Developed under the direction of Dr. Neil Bander at Weill Cornell Medicine
-
Treatment contains two different radioactive drugs
- Radioactive antibody - 225Ac-J591 (aka CONV 01-α) with Actinium
- Radioactive small molecule - 177Lu-PSMA I&T with Lutetium
Clinical Trials

-
Phase 1 data on 225Ac-J591 presented at ASCO June 4, 2021
https://meetinglibrary.asco.org/record/196409/abstract - Phase 1b / 2a Multiple Ascending Dose 225Ac-J591 ongoing
- Phase 1b / 2a dual therapy trial now approved to proceed by FDA
- Additional dual drug human trials
June 4, 2020 225Ac-J591 Phase I Data
- PSA: decline in 68.8%; >50% decline in 43.8%; 13 of 16 at highest dose
- Circulating tumor cells: decline in 50% (5/6 at highest dose); 27% stayed undetectable
- 32 Patients: mCRPC with prior potent chemo/radiotherapy
- Single dose of 225Ac-J591, seven dose groups, 16 at highest dose
- No dose limiting toxicity in 16 patients at highest dose (1 in 2nd highest)
Conclusions:
- Drug is “well tolerated”
- Decline in PSA and CTC in heavily pretreated population
- Neal Shore, MD, FACS, a well-known key opinion leader in prostate cancer research:
“These Phase I trial results of the 225Ac-J591 antibody … are impressive, both from a response and safety standpoint. PSA declines and CTC responses were clearly evident.”
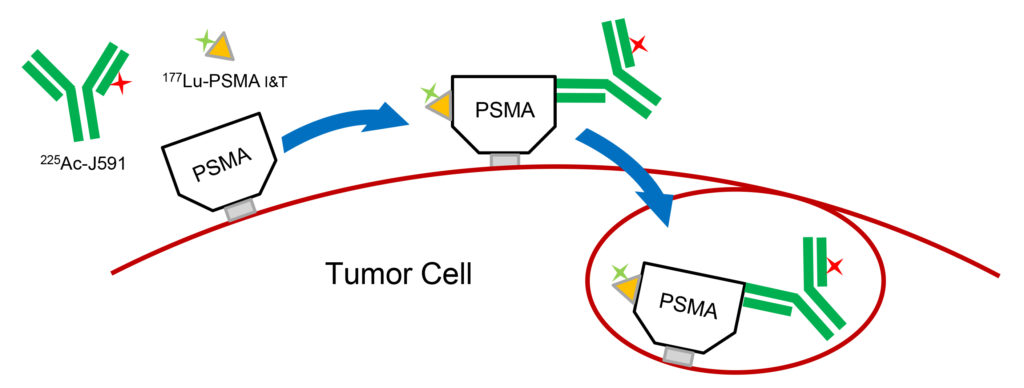
How Does the Dual Therapy Work?
- Both drugs bind to PSMA receptor noncompetitively
- Antibody causes internalization of both drugs
- Direct insertion of two different drugs
- Non-overlapping biodistributions in the body-reduces additive damage
Radiotherapy Monetization Datapoints
-
Lutathera® (177Lu-Dotatate) – $3.9B acquisition of AAA by Novartis in October 2017
https://www.fiercepharma.com/pharma/novartis-snags-blockbuster-hopeful-lutathera-3-9b-deal-for-advanced-accelerator -
177Lu-PMSA-617 – $2.1B license by Novartis from Endocyte in October 2018
https://www.fiercebiotech.com/biotech/novartis-inks-2-1b-endocyte-buyout-furthering-radiotherapy-push -
Positive Phase III data in advanced prostate cancer announced by Novartis in March 2021
https://www.novartis.com/news/media-releases/novartis-announces-positive-result-phase-iii-study-radioligand-therapy-177lu-psma-617-patients-advanced-prostate-cancer -
177Lu-PSMA I&T for dual therapy very similar to Novartis 177Lu-PMSA-617
- Same PSMA binding structure
- Same 177Lu binding structure
Other Radiotherapy Transactions
-
6/4/2021: Point Biopharma – 177Lu small molecule drug similar to Novartis’s drug
- Dosing underway in Phase 3 SPLASH study for mCRPC with drug PNT2002
- Upon closing with Research Alliance (NASDAQ: RACA) will go public under ticker PNT (mid-2021)
- Combined company expected to have initial equity value of ~$924M
- https://www.pointbiopharma.com/press-releases
-
6/4/2021: Bayer acquired Noria Therapeutics and PSMA Therapeutics 6/4/2021
- Planning small molecule drug with 225Ac for mCRPC
- https://www.biospace.com/article/bayer-acquires-noria-psma-therapeutics-to-expand-place-in-prostate-cancer-space/
-
11/2/2020: Astrazeneca partnered with Fusion Pharmaceuticals
- Fusion currently recruiting in Phase I on 225Ac-FPI-1434, an antibody drug for solid tumors
- https://www.sec.gov/Archives/edgar/data/1805890/000119312520283319/d98805dex991.html
- In 2020, Fusion raised combined >$350M in venture funding and IPO
- https://ir.fusionpharma.com/news-releases
-
Global radiopharmaceutical market: ~$3.6B in 2018 projected to ~$5.4B by 2027; CGAR~4%
